Description
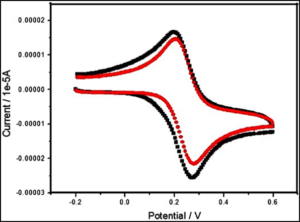
Nanomaterials are revolutionizing fields like electronics, catalysis, and energy storage. One key technique for studying these materials is cyclic voltammetry (CV), an electrochemical method that examines the redox properties of materials by measuring current while sweeping voltage linearly back and forth. CV measures the current in an electrochemical cell as the voltage is swept. The resulting plot, called a cyclic voltammogram, reveals vital information about the material’s redox behavior, electron transfer kinetics, and stability.
Key Parameters
- Peak Current (Ip): Indicates the rate of the redox reaction.
- Peak Potential (Ep): Shows the energetics of the redox process.
- Anodic and Cathodic Peaks: Correspond to oxidation and reduction processes.
Applying CV to Nanomaterials
Nanomaterials, like gold nanoparticles, graphene, carbon nanotubes, and titanium dioxide, have unique electrochemical properties. CV helps in studying their size, shape, surface chemistry, electron transfer kinetics, and catalytic activity.
Examples:
- Gold Nanoparticles (AuNPs): Used in catalysis and sensors CV reveals their redox behavior.
- Graphene and CNTs: Used in energy storage. CV studies their electron transfer and functionalization effects.
- Titanium Dioxide (TiO2): Used in photocatalysis and solar cells. CV helps optimize charge transfer processes.
Advantages of CV
- Versatility: Applicable to various materials.
- Detail: Provides in-depth information on redox processes.
- Rapid Analysis: Quick and straightforward.
For does this analysis please provide information below:
- 50 ml of electrolyte at least to perform the test.
- The sample area is measurable.
- Powder samples should be prepared in tablet form. If you cann’t do this, the required amount of sample must be sent.
- Thin layer samples must have a conductive back contact.
- The sample size should be at least 1.5 cm * 1 cm.
Information of samples:
- The nature of the sample must be stated.
- The type of electrolyte must be announced.
- The voltage scan speed must be announced in mV/s.
- The frequency range must be declared in Hz.
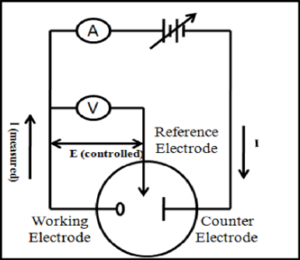
- The type of reference electrode and counter electrode should be determined.
- Carefully select the type of analysis requested and, if possible, submit an article similar to the executive protocol.
A- Electrochemical impedance spectroscopy (EIS)
B- cyclic voltammetry (CV)
C- chronoamperometry (CA)
D- chronocoulometry (CC)