Description
The development of gas sensors currently is being carried out intensively, this is largely in part due to environmental pollution and toxicants found the atmosphere of both industrial and domestic settings. This pollution and toxicants found in the atmosphere represent acute problems facing mankind.
Gas detection is obligatory in many different fields, e.g., industrial, fuel emission control, automobile exhaust emission control, household security, and environmental pollution monitoring. Gas sensors are utilized in factories, laboratories, hospitals, and almost all technical installations.
Gases of interest include CO2, CO, NO2, SO2, O2, O3, H2, Ar, N2, NH3, and organic vapors such as methanol (CH3OH), ethanol (C2H5OH), isopropanol (C3H8O), benzene (C6H6), and some amines (organic compounds and functional groups that contain a basic nitrogen atom with a lone pair).
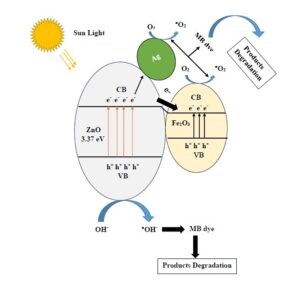
The mechanisms for recognizing the gases to be determined are the absorption processes, e.g., in metal oxides (MOXs) and carbon nanotubes (CNTs), and specific recognition for the formation of supramolecular (large molecules formed by grouping or bonding several molecules together) or covalent bonds between the sensor and the analyte, as in some metal complexes.
Studies have revealed that sensitivity increases or response time decreases as the film thickness or the particle size of MOXs or organic polymers decreases.
A characteristic feature of gas nanosensors is that they have a transducer system consisting of nanometric materials. On this basis, there are two kinds of devices:
- In some devices, the sensor and the transducer are different components.
- In other devices, the sensor also functions as the transducer.
Sensor Parameters
To characterize the quality of the output signal produced by a sensor, some specific parameters are generally used. The most important parameters are intensity, reversibility, response and recovery times, sensitivity, specificity, selectivity, stability, and dynamical range.v
1.Sensitivity: The response of a sensor upon the introduction of a particular gas species is called the sensitivity (S). The most general definition of sensitivity applied to gas sensors is a change in the electrical resistance (or conductance) relative to the initial state upon exposure to a reducing or oxidizing gas component) [1]. A common approach to report S is shown in the equations below.
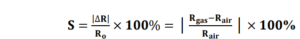
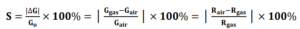
Where R is the electrical resistance, G is the electrical conductance, and the subscript “air” indicates that the background is the initial dry air state, and the subscript “gas” indicates the analyte gas has been introduced.
- Selectivity is defined as the ability to discriminate a particular gas species from the background atmosphere. This is a task where metal oxide-based sensors face significant challenges and show poor discrimination between gas species. Selectivity has been defined as the ratio of sensitivities to a particular gas as shown in equation.
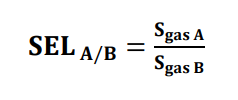
The selectivity of a gas sensor should be always greater than one.
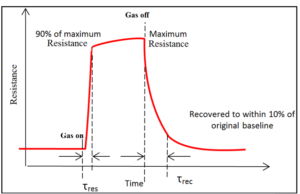
- Response and Recovery Times: The response time is the time interval over which conductance attains a 90%of final value when the sensor is exposed to target gas. Recovery time is the time interval over which sensor conductance reduces to 10% of the saturation value when the sensor is the case of gas off and then placed in clean air, as shown in Figure (1). A good sensor should have a small response and recovery times so that the sensor can be used repeatedly.